
Table of contents:
- Author Sierra Becker becker@designhomebox.com.
- Public 2024-02-26 03:46.
- Last modified 2025-01-22 22:09.
Chemistry has endowed mankind with a mass of useful compounds, greatly facilitating life and opening up many new areas previously unknown to people. Among the necessary substances is sodium sulfite, which has found its application in a wide variety of branches of human activity.

Chemical and physical properties
Sodium sulfite (anhydrous) is a white powder, sometimes with a yellowish tinge. Does not burn, does not have the ability to explode, but when heated, it decomposes, forming toxic gases, in connection with which it was assigned to the 3rd hazard class. The decomposition products that sodium sulfite forms can disrupt the central nervous system, cause fainting if inhaled, reduce the ability to breathe, excessively accelerate the heart rate, and cause damage to bones, skin and eyes. That is why in the event of a fire, where sodium sulfite is stored, it is necessary to enter it as protected as possible: in a special suit and always with a breathing apparatus. If the substance is scattered, this place must be protected with a side of the earth, the powder itself should be covered with something neutral (for example, sand) and only after thatcollect.
Sodium sulfite - preservative
Where is this powder used? The substance has very useful chemical properties. Thanks to them, sodium sulfite is used, for example, in the food industry. Fruits and vegetables processed with it are stored longer without darkening. As a preservative, it is used in winemaking and in the manufacture of sweets; it is used to make long-stored dried fruits. At the same time, it should be noted that in Germany it is forbidden to use sodium sulfite in the processing of meat, since it masks its stale color, which can lead to mass poisoning.

Other applications
In addition to food, the second main use of this compound is textile, as well as pulp and paper production. This is where the main amounts of sodium sulfite go. But it is also used for water purification, and for leather dressing. It purifies trinitrotoluene, which is subsequently used in mining or for military purposes. Pharmaceutics and medicine also do not neglect this substance. Here, a solution of sodium sulfite is often needed. The compound referred to here is also used in the production of non-ferrous metals and sodium thiosulfate, which is prescribed by doctors in cases of poisoning with derivatives of lead, mercury and arsenic.
Obsolete direction

In the days of film cameras and film cameras, sodium sulfite was simply necessary for developing films themselves, preventing oxidation of solutions and washing media (films orphoto paper) from the fixer. Now, with the spread of digital analogues, this use of this substance has remained mainly for amateurs who have retained the old technique. Specialists-photographers of the old generation claim that when developing black-and-white films, it is sodium sulfite that makes it possible to achieve the most traced details in the shadows and significantly increases the photosensitivity with unsuccessful negative contrast.
As you can see, this is a substance that is necessary in various areas of human life. And if some direction of its use becomes irrelevant, another, no less important one is found.
Recommended:
African mask and its magical meaning

Recently, the habit of decorating home interiors with masks has come into fashion: they are brought from exotic travels, bought in stores. Perceiving masks as a symbol of costumed masquerades, they are not taken seriously enough
How to draw a chessboard and its pieces
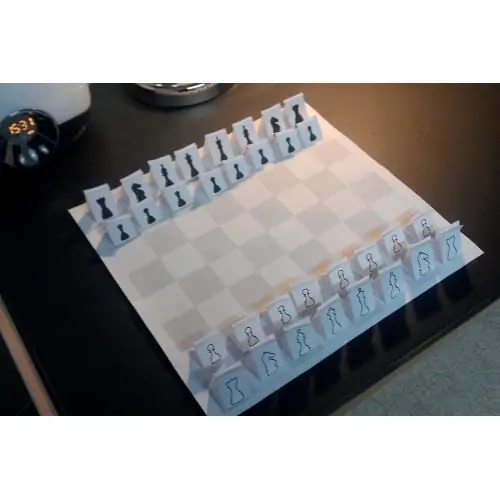
Chessboard is a beautiful and very irreplaceable thing. She may not be at home for various reasons - inability to play, financial situation. But sometimes there comes the very case when her presence is simply necessary. This article describes how to draw a chessboard, how to decorate it beautifully with the help of improvised means, and how to make beautiful figures for the game
Knitted vest and its benefits

Many people have a knitted vest. Someone buys it as a product that allows you to look more stylish. Most people prefer this item due to its functionality
How to knit a carrot knot correctly. Its main advantages and disadvantages

This mount is quite strong and compact, the knot easily passes through the rings of feeder and carp rods. Its resistance is increased due to the thickened site of the node. That is why it is so popular among fishermen. However, it is necessary to understand in detail what it is and how to knit a carrot knot
Crochet slip stitch: basic knitting principles and uses

Crochet neat and tidy for a professional look. To do this, you will need a sliding crochet loop
